The EBL2005 clinical trial (EBOVAC Infant Study) is a Phase 2 Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of the Heterologous 2-dose Vaccination Regimen Using Ad26.ZEBOV and MVA-BN®-Filo in Infants Aged 4-11 Months in Guinea and Sierra Leone.

Rationale & Experimental design
This Phase 2 study aims to improve preparedness for future Ebola outbreaks by vaccination of a new, at-risk population. This study will be conducted in healthy infants aged 4 – 11 months (ie, ≥4 months up to <12 months) (referred to as ‘infants’) in Guinea and Sierra Leone. This study will expand the safety, reactogenicity, and immunogenicity database for VAC52150, to infants vaccinated with a heterologous 2-dose regimen with Ad26.ZEBOV followed by MVA-BN-Filo administered 56 days later.
This study will build on earlier studies with the candidate Ebola vaccines in older children aged 1 to 17 years (EBL2002: 4-11 and 12-17 years; EBL3001: 1-3, 4-11, and 12-17 years). Though the incidence of Ebola virus disease in younger children is low, it has been reported that the mean incubation period (the average time from infection until symptom onset) in children was shortest on average in the youngest children, with means ranging from 6.9 days (95% confidence interval [CI], 5.1-9.5) in 14 children younger than 1 year of age, to 9.8 days (95% CI, 8.7-11.1) in 184 children 10-15 years of age. Younger children also had shorter times from symptom onset to hospitalization and from symptom onset to death, and the highest mortality rate. It is therefore important to evaluate any vaccine for Ebola for safety and immunogenicity in this population.
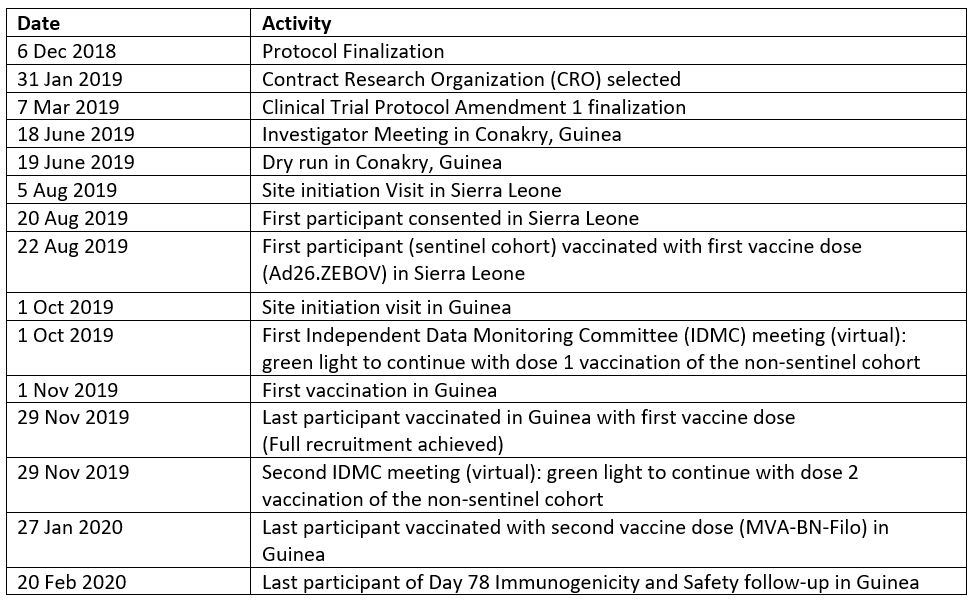
Study activities




Collaborators and partners in the EBOVAC-Salone Extension Study include:
- Janssen Vaccines & Prevention B.V.
- London School of Hygiene and Tropical Medicine
- Institut National de la Santé et de la Recherche Médicale
- College of Medicine and Allied Health Sciences, University of Sierra Leone
- Coalition of Epidemic Preparedness Innovations



