
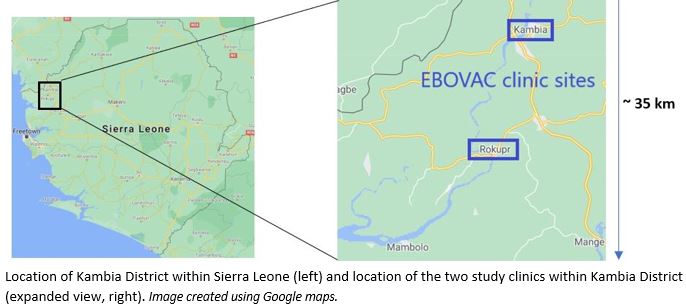
The EBOVAC-Salone Extension Study (EBL3005) is designed to prolong the follow-up of participants in a previous study (the EBOVAC-Salone trial-EBL3001) to a total period of four to five years for each participant.
In the EBOVAC-Salone trial, under EBOVAC1, participants were vaccinated with either: (a) the 2-dose Ebola vaccine regimen of Ad26.ZEBOV followed by MVA-BN®-Filo administered 56 days later; or (b) a quadrivalent meningococcal vaccine. They were then followed up for one year (children, aged 1 to 17 years), or for two to three years (adults). The participants who received at least one Ebola vaccine in the EBOVAC-Salone trial are eligible to take part in the EBOVAC-Salone Extension Study. The purpose of the EBOVAC-Salone Extension Study is to evaluate the long-term safety and immunogenicity of the two-dose vaccine regimen, up to four years after vaccination (in those aged 1 to 17 years at the time of vaccination), or up to five years after vaccination (in adults).
The Extension study also includes children of female EBOVAC-Salone participants, who were conceived either (a) during the 3-month period following vaccination with Ad26.ZEBOV, or (b) during the 28-day period following vaccination with MVA-BN®-Filo.
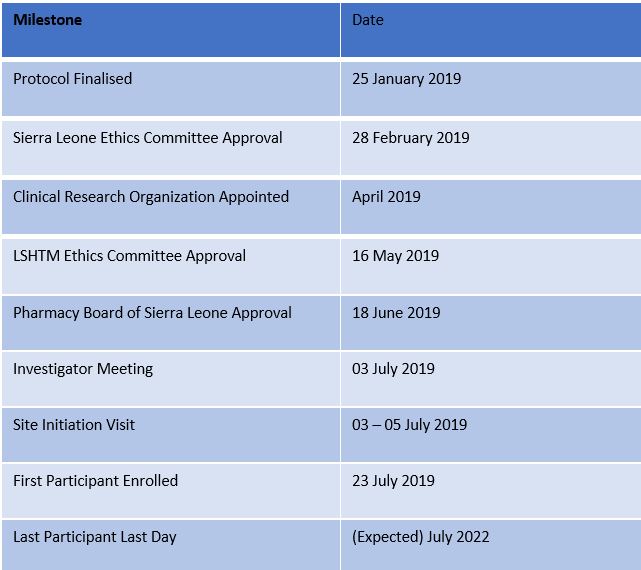
Study Activities


Collaborators and partners in the EBOVAC-Salone Extension Study include:
- Janssen Vaccines & Prevention B.V.
- The London School of Hygiene and Tropical Medicine
- The College of Medicine and Allied Health Sciences, University of Sierra Leone



